Stability Studies
Scientific expertise
According to ICH Q1A and Q5C guidelines
20 Years of experience
Stability studies play a vital role in the development of drug products. According to the ICH Q1A guidelines, “the purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental factors such as temperature, humidity, and light.” This helps to establish the shelf life of a drug product during storage, suitable storage conditions, and a re-test period for the drug substance. Notably, biologics, biosimilars, viruses, vaccines, and biofunctionalized devices are especially susceptible to the influence of environmental factors during processing, transport, storage, or handling.
Stability studies evaluate the stability of a DS or DP under various conditions such as temperature, light, or mechanical stress over time. An evaluation encompassing both in-vitro and in-silico methods as an integrated approach enables a comprehensive understanding of the product's stability profile. In-silico methods can give you prediction power which can shorten your development timelines. Leukocare specializes in providing these advanced testing and prediction techniques. Our team performs stability studies according to ICH Q1A guidelines and can support the data set for registration applications.
Stability Study Expertise
With more than 20 years of scientific and regulatory experience, and guided by the internationally recognized ICH Q1A-E guidelines, our team executes stability studies with precision, generating robust data that not only assures product stability but also serves as a valuable asset for registration applications.
Stability testing services cover temperature stress (Freezer and ICH Q1A storage cabinets covering temperatures from -80 °C to +40 °C), light stress to assess photostability (ICH Q1B light tester) and various models of mechanical stress. With a broad analytical toolbox optimized for stability indicating analyses of the CQAs, we evaluate the stability at specific time points. For long-term stability prediction, we can provide regression analysis (linear and multiple) and kinetic modeling based on either in-house stability data or data from your GMP laboratory. With kinetic modeling, we can even extend the quality of extrapolation even beyond ICH Q1E guidelines.
In essence, stability studies stand as a cornerstone of our commitment to delivering drug products that endure the test of time and environmental challenges.
- Real-time, intermediate and accelerated stability storage (non-GMP)
- Long-term stability studies (non-GMP)
- In-use stability studies
- Stability-indicating analytics
- Linear and multiple regression analysis for long-term shelf-life prediction
- Kinetic Modeling for long-term shelf-life prediction
Case study - Accelerated aging
Predictive power of accelerated aging
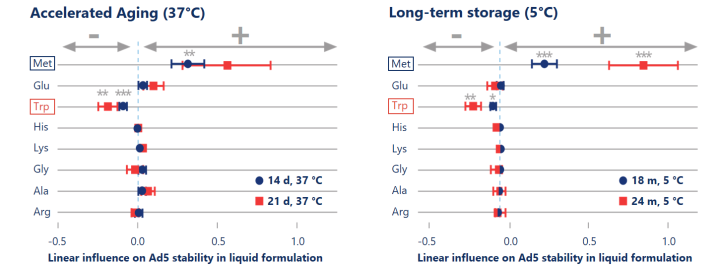
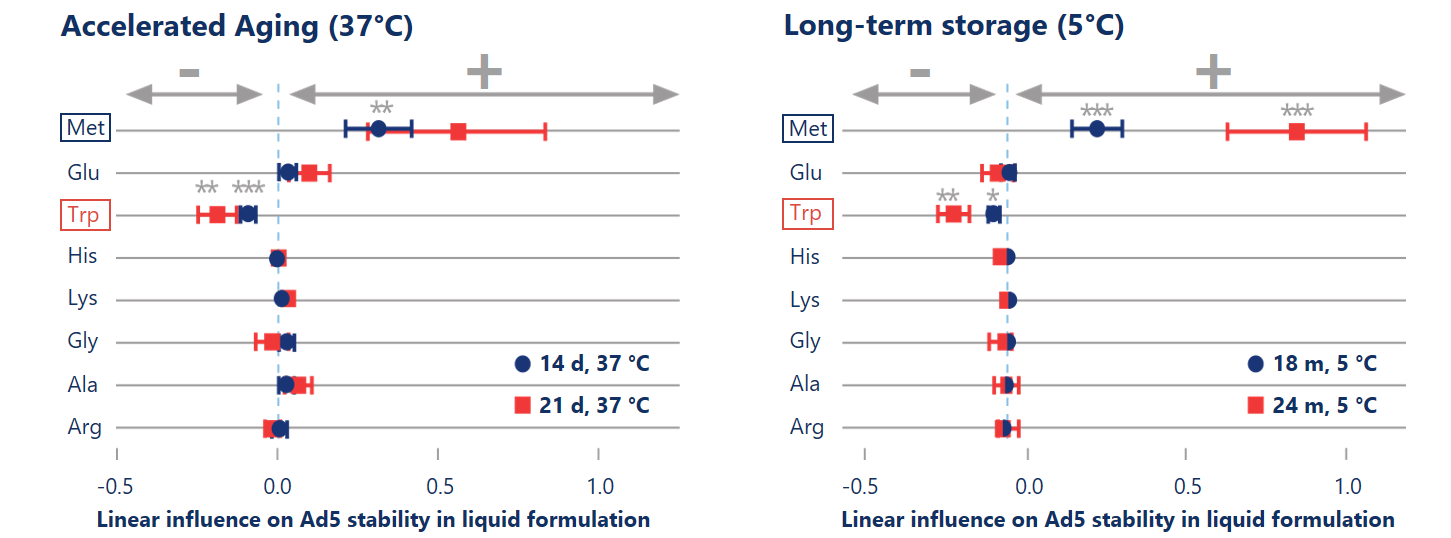
Combination of accelerated aging and DoE speeds up the formulation development process.
Design of Experiments (DoE) based formulation development allows to apply regression analysis to statistically estimate the stabilizing effects of excipients. Accelerated aging at high temperature (37 °C, left) showed predictive power in detecting statistically significant (p< 0.01) stabilizing effects of methionine on the titer.
Reinauer et al. (2020), J Pharm Sci
Kinetic Modeling
Kinetic Modeling Provides Better Prediction Accuracy
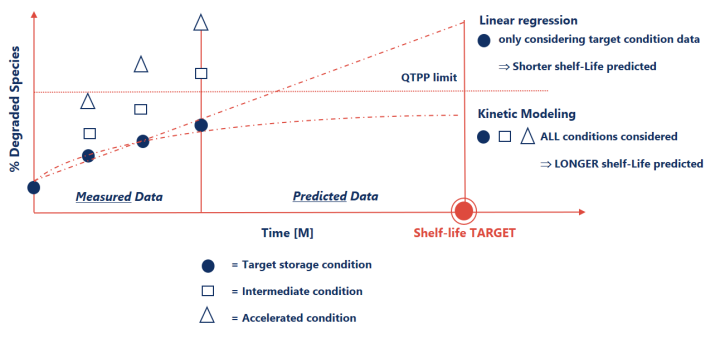
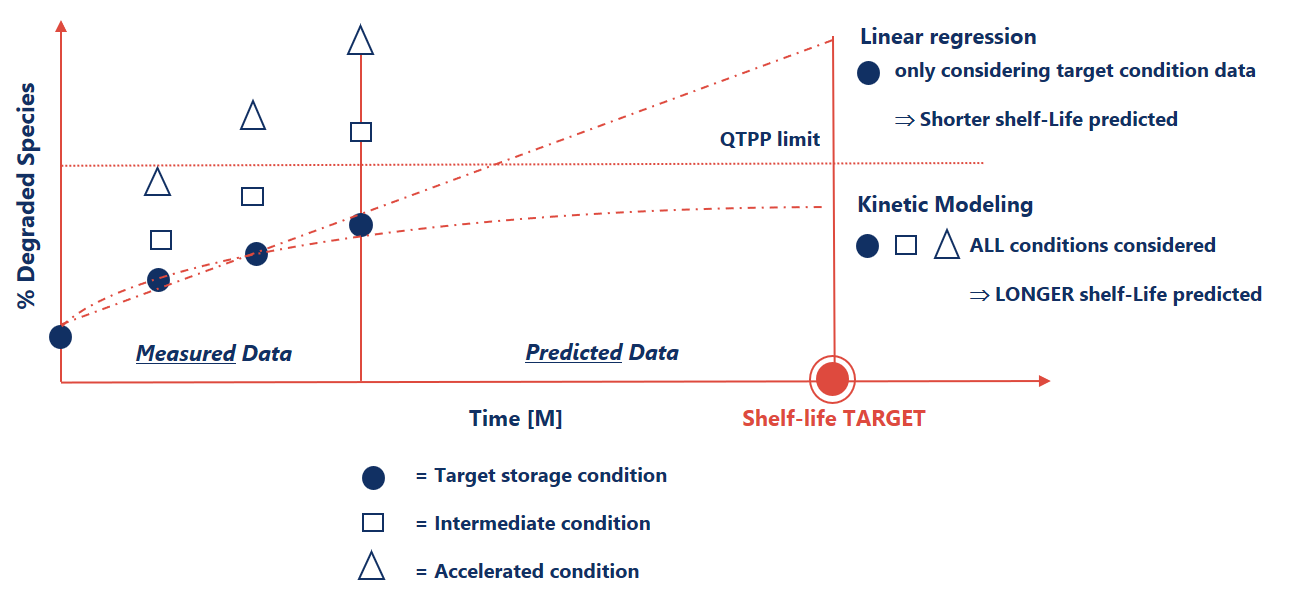
Shelf-life prediction as an enabler for faster decision making
Current ICH guidelines support the use of Linear Regression as golden standard to predict shelf life. However, Kinetic Modeling can lead to more accurate stability predictions of DS such as antibodies and viral vectors that exhibit complex multi-step degradation patterns and autocatalytic behavior.
Contact us
Ensure your drug product stability using in-vitro and in-silico analytical methods.
Contact usPoster - Kinetic Modeling
See why Kinetic Modeling shows higher accuracy in shelf-life predictions of antibodies and viral vectors
Webinar - AAV Stability Assessment
Stability Assessment in Drug Product Formulation Development